
The ANDA process is the backbone of affordable generic drugs in the United States. It’s how companies get approval to sell copies of brand-name medications without repeating expensive clinical trials. But behind this streamlined system lies a complex web of legal, scientific, and regulatory rules. If you’re trying to bring a generic drug to market, you can’t just copy the pill and call it done. The FDA demands proof-rigorous, detailed, and legally binding-that your version works exactly like the original. Failure to meet these standards means rejection, delays, or even lawsuits. This isn’t bureaucracy for bureaucracy’s sake. It’s the law, designed to protect patients while keeping prices low.
What the ANDA Process Actually Is
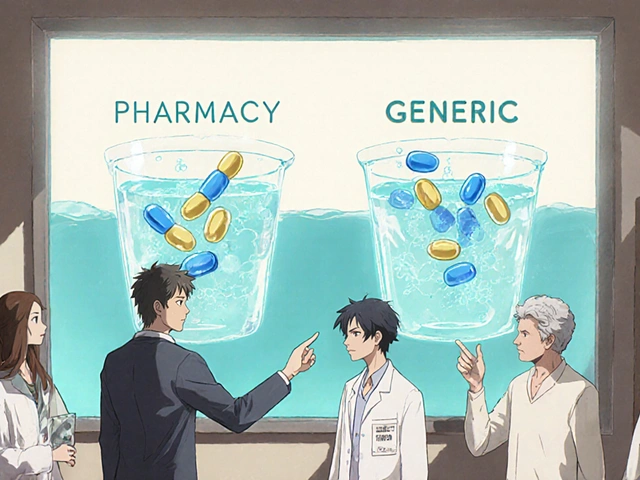
The Abbreviated New Drug Application, or ANDA, is a legal pathway created by the Hatch-Waxman Act of 1984. That law didn’t just make things easier for generic manufacturers-it struck a balance. It gave brand-name companies enough patent protection to recoup their R&D costs, while giving generic makers a clear route to enter the market once those patents expired. The word "abbreviated" is key. Unlike a New Drug Application (NDA), which requires years of clinical trials to prove safety and effectiveness, an ANDA skips all that. Instead, it relies on the FDA’s prior approval of the brand-name drug, called the Reference Listed Drug (RLD). Your job? Prove you’re the same.
This isn’t a loophole. It’s a legally recognized shortcut. The FDA doesn’t need to re-test every drug every time. That’s why 90% of prescriptions in the U.S. are filled with generics today. And it’s why Americans have saved over $2.2 trillion in drug costs over the last decade. But that savings only happens if every generic meets the exact same legal standards.
Core Legal Requirements for ANDA Approval
To get an ANDA approved, you must satisfy five non-negotiable legal requirements set by the FDA. Missing even one can trigger a refusal to receive your application-or worse, a complete rejection after months of review.
- Identical Active Ingredient: Your drug must contain the exact same active pharmaceutical ingredient (API) as the RLD. No substitutions. No "similar" versions. If your API has a different salt form, crystal structure, or impurity profile, you need a suitability petition under Section 505(j)(2)(C) of the FD&C Act. That’s a separate, harder process.
- Same Dosage Form, Strength, Route, and Use: If the brand is a 10mg tablet taken orally once daily for high blood pressure, your generic must be identical. No changing the pill to a capsule. No altering the dose. No adding new indications. The FDA’s labeling requirements are strict here. You can only change the manufacturer’s name and the "generic" disclaimer.
- Proven Bioequivalence: This is where most applications fail. You must run pharmacokinetic studies showing your drug is absorbed into the bloodstream at the same rate and to the same extent as the RLD. The FDA requires 90% confidence intervals for Cmax (peak concentration) and AUC (total exposure) to fall between 80% and 125% of the brand’s values. These aren’t suggestions-they’re legal thresholds. Studies must follow FDA guidance (2023 version), use validated methods, and include healthy adult volunteers under controlled conditions.
- Identical Labeling: Your package insert, warnings, and instructions must match the RLD’s, except for minor edits to reflect your company as the manufacturer. You can’t add new contraindications or remove safety warnings. Doing so could be seen as misbranding under the FD&C Act.
- Comprehensive CMC Documentation: Chemistry, Manufacturing, and Controls (CMC) data is the most overlooked-and most rejected-part of ANDA submissions. You must detail every step: raw material sourcing, manufacturing process, equipment used, quality control tests, stability studies, and container closure systems. The FDA inspects your facility. If your cGMP compliance is weak, your application dies.
Submission Rules and Fees
Submitting an ANDA isn’t just about sending documents. It’s about following a rigid legal format. All applications must be in Electronic Common Technical Document (eCTD) format. Paper submissions are rejected outright. You must include Form FDA-356h (the official application form) and FDA-3674 (user fee cover sheet). Missing either means your application won’t even be reviewed.
There’s also a financial cost. For fiscal year 2024, the user fee for an original ANDA is $129,500. A supplement (like changing a supplier or packaging) costs $5,000. These fees fund the FDA’s review process under the Generic Drug User Fee Amendments (GDUFA). Paying isn’t optional-it’s a legal requirement tied to the approval timeline. If you don’t pay, your application is put on hold indefinitely.
And you can’t just test a small batch. The FDA requires exhibit batches made at commercial scale: at least 10% of your planned commercial batch size-or 100,000 dosage units, whichever is larger. These batches must be stable, properly labeled, and stored under real-world conditions for at least 12 months before submission.
Patent Certification and Legal Risks
One of the most legally complex parts of the ANDA process is patent certification. When you file, you must choose one of four certifications:
- Paragraph I: The patent information hasn’t been submitted to the FDA.
- Paragraph II: The patent has expired.
- Paragraph III: You’ll wait until the patent expires.
- Paragraph IV: The patent is invalid or won’t be infringed.
Paragraph IV is the most dangerous-and the most common. Filing a Paragraph IV certification is essentially a legal challenge. The brand-name company can sue you for patent infringement, triggering a 30-month stay on FDA approval. That’s not a delay-it’s a legal freeze. Many companies get stuck in litigation for years, even if their drug is perfectly bioequivalent. According to industry reports, one Paragraph IV filing can add 41 months to the timeline. And if you lose the lawsuit? You’re barred from selling your generic for the life of the patent.
Why Many ANDAs Get Rejected
According to FDA data, 58% of first-time ANDA submissions receive a deficiency letter. The top reasons? Incomplete bioequivalence protocols (28%) and inadequate CMC data (23%). These aren’t minor errors. They’re legal failures.
One company submitted an ANDA for a generic nasal spray. Their bioequivalence study used the wrong device calibration method. The FDA refused to receive the application. Another company skipped validating their container closure system. Their generic was rejected because the packaging could affect drug stability. These aren’t edge cases-they’re routine.
Even small mistakes matter. A missing signature. A typo in the batch number. A stability study that didn’t cover all storage conditions. The FDA’s Refuse-to-Receive standards list 147 specific criteria. One violation, and your application is tossed without review.

How to Increase Your Chances of Approval
The path to approval isn’t random. Companies that succeed do three things right:
- Use pre-ANDA meetings: In 2022, the FDA held 1,842 of these meetings. They’re free, confidential, and invaluable. You can ask the FDA what they expect before you spend millions. Many companies skip this and pay the price later.
- Hire experienced regulatory staff: Regulatory affairs professionals with RAC certification earn median salaries of $125,000. That’s not an expense-it’s insurance. A single expert can prevent a rejection that costs $10 million in lost time.
- Test early, test often: Don’t wait until submission to run bioequivalence studies. Run them during development. Use the same manufacturing equipment you’ll use commercially. If your tablet is made on a different press than the brand’s, you need to prove it doesn’t change absorption.
Some companies succeed quickly. Lupin Limited got approval for a generic version of Jardiance in just 9.5 months. Their secret? A "clean application"-complete CMC data, perfect bioequivalence, no missing forms. Others, like Teva, spent 42 months and $28 million on a single ANDA for a complex inhaler. The difference? Preparation.
The Bigger Picture: Why This Matters
The ANDA process isn’t just about paperwork. It’s about access. Generic drugs make life-saving medications affordable. A 30-day supply of brand-name Lipitor might cost $300. The generic? $10. That’s the power of competition. But that competition only works if the rules are enforced. The FDA doesn’t approve generics to be cheap. They approve them to be safe, effective, and identical.
Right now, 35% of pending ANDAs are for complex products-inhaled drugs, topical creams, injectables. These are harder to replicate. The FDA’s approval rate for these is only 42% on first review. That’s why the agency is investing $15 million in 2024 to develop better scientific tools. The goal? Faster, fairer approvals without lowering standards.
Legislation like the CREATES Act of 2019 is also helping. It stops brand companies from blocking access to samples needed for testing. Without it, some generics can’t even start development.
The ANDA process is a legal machine. It’s precise, unforgiving, and powerful. Get it right, and you bring down drug prices. Get it wrong, and you waste millions. There’s no middle ground.
What is the difference between an ANDA and an NDA?
An NDA is for brand-name drugs and requires full clinical trials to prove safety and effectiveness. An ANDA is for generics and relies on the FDA’s prior approval of the brand-name drug. Instead of new clinical data, an ANDA proves bioequivalence and manufacturing consistency. NDAs take 10-15 years and cost over $2 billion. ANDAs take 3-5 years and cost $5-10 million.
Can I change the formulation of a drug in an ANDA?
No, not without a suitability petition. The ANDA requires the generic to be identical in active ingredient, dosage form, strength, route, and use. If you want to change the inactive ingredients, tablet shape, or delivery method, you must file a petition under Section 505(j)(2)(C). The FDA may deny it if the change affects safety or effectiveness. Most companies avoid this route and use the 505(b)(2) pathway instead.
How long does the ANDA review process take?
Under GDUFA III (2023-2027), the FDA aims to review standard ANDAs in 10 months and priority ANDAs in 8 months. But in practice, the average is 30-36 months due to deficiency letters, complex products, and patent litigation. Simple generics can be approved faster; complex ones like inhalers or injectables often take over 40 months.
What happens if my ANDA gets rejected?
If the FDA issues a refusal to receive, your application is thrown out before review. If you get a complete response letter, you have 180 days to fix the issues and resubmit. Each resubmission restarts the clock. Many companies spend millions and years trying to fix one application. Pre-submission meetings and expert consultants can reduce this risk.
Do I need to inspect my manufacturing facility before submitting an ANDA?
Yes. The FDA inspects all manufacturing sites before approving an ANDA. Facilities must comply with Current Good Manufacturing Practices (cGMP). Overseas facilities are inspected just as often as U.S. ones. In 2022, 68% of FDA Form 483 observations were at foreign plants. If your facility has unresolved violations, your ANDA will be delayed or denied.




Colin L
December 31, 2025 AT 04:30The ANDA process is a masterclass in regulatory overreach disguised as patient protection. You think this is about safety? Nah. It's about protecting Big Pharma's oligopoly under the guise of science. The 80-125% bioequivalence window? That’s a joke. Two drugs can be wildly different in dissolution profile and still pass. The FDA doesn’t care as long as the numbers look pretty on paper. And don’t get me started on the $130K fee - that’s a tax on competition, not a user fee. The real winners? Lawyers who specialize in Paragraph IV challenges. They’re the ones making bank, not patients.
Hayley Ash
January 1, 2026 AT 16:22So let me get this straight - you can’t change the tablet shape but you can change the color? That’s the law now? 🤡
Nadia Spira
January 2, 2026 AT 12:05Let’s be real - this entire system is a performative farce. The FDA doesn’t have the bandwidth to meaningfully inspect 68% of foreign facilities. The cGMP checks are theater. The bioequivalence studies? Often run by CROs with zero accountability. And the so-called "identical labeling"? That’s a legal fiction. Warnings are buried in 200-page inserts. Patients don’t read them. The system doesn’t protect anyone - it just makes generic manufacturers jump through hoops so Big Pharma can keep pricing power. It’s capitalism with a lab coat.
Kunal Karakoti
January 4, 2026 AT 09:59There’s a quiet beauty in how the ANDA process balances innovation with access. It’s not perfect, but it’s one of the few systems where law, science, and economics converge to serve the public good. The fact that 90% of prescriptions are generics isn’t an accident - it’s the result of deliberate policy. The delays, the fees, the inspections - they’re the cost of trust. We could have cheaper drugs, but we’d lose confidence. And confidence, in medicine, is the most valuable ingredient of all.
Kelly Gerrard
January 5, 2026 AT 17:24Every single one of these requirements exists for a reason. If you can’t meet them, you shouldn’t be in pharma. End of story.
Glendon Cone
January 6, 2026 AT 03:18Big props to the FDA for keeping this system from collapsing. I’ve seen too many startups think they can skip CMC data and just "wing it." Spoiler: they get rejected. Hard. The 10-month review clock? That’s a miracle considering how many submissions are half-baked. Also - pre-ANDA meetings are a game-changer. Do them. 🙏
Henry Ward
January 8, 2026 AT 01:55Anyone who thinks this system is fair is delusional. The companies that succeed? They have lawyers on retainer, consultants on payroll, and offshore labs with no oversight. Meanwhile, small innovators get crushed under the weight of $130K fees and 3-year delays. This isn’t regulation - it’s a tax on the poor. And the FDA? They’re just the enforcers of a rigged game.
kelly tracy
January 9, 2026 AT 20:26Oh wow. Another love letter to bureaucratic hell. The FDA is a monster. A slow, expensive, soul-crushing monster. And you people actually think this is necessary? You’re the reason generics cost more than they should. Wake up.
Aayush Khandelwal
January 9, 2026 AT 21:51Man, the ANDA system is like a Bollywood drama - all drama, no resolution. You spend 3 years, $20M, and then some FDA inspector in Rockville finds a typo in Form 356h and says "nope." Meanwhile, the same guy approves a bioequivalence study from a lab in Mumbai that uses a centrifuge from 1998. It’s not about standards - it’s about who you know. And the worst part? The patients still pay the price.
Sandeep Mishra
January 10, 2026 AT 17:31For those new to this: the ANDA process isn’t a wall - it’s a bridge. It’s designed to let you cross from idea to market without drowning in clinical trials. The hurdles? They’re there because lives are on the line. I’ve seen what happens when generics aren’t identical - heart arrhythmias, seizures, overdoses. The system is rigid because medicine can’t afford flexibility. Be patient. Be thorough. The system rewards those who respect it.
srishti Jain
January 10, 2026 AT 21:50lol the FDA is a joke. I’ve seen ANDAs get rejected for font size.
Shae Chapman
January 12, 2026 AT 09:25Just want to say huge respect to the regulatory teams grinding through these submissions. You’re the unsung heroes keeping our meds safe and cheap. 🫶 I know it’s stressful but you’re doing god’s work. And hey - if you’re reading this and you’re in the trenches - you’re not alone. We see you.
henry mateo
January 13, 2026 AT 15:04im not a lawyer or a scientist but i think the 80-125% thing is wild. like what if your drug works better but just has a slightly different absorption? why is that not allowed? also why do we even need to pay $130k? who gets that money?
Joseph Corry
January 14, 2026 AT 11:55Let’s not romanticize the ANDA process. It’s not a triumph of public health - it’s a carefully constructed legal architecture designed to delay competition until the patent expires, then let generic manufacturers fight over crumbs while the original brand cashes out on evergreen patents. The 30-month stay? That’s not a delay - it’s a strategic pause engineered by patent trolls and pharma lobbyists. The FDA’s role? To serve as the referee in a game rigged from the start. The real innovation isn’t in the pills - it’s in the legal loopholes. And those are the only things that move fast.
Cheyenne Sims
January 16, 2026 AT 01:53There is no justification for the systemic failure of the ANDA review timeline. The FDA’s own GDUFA metrics state a 10-month review window. The reality is 30 to 36 months. That is not inefficiency - that is institutional negligence. The user fees are collected. The resources are allocated. Yet the system remains broken. This is not a regulatory challenge - it is a failure of leadership. Accountability must be demanded. Transparency must be enforced. And the patients - the very people this system claims to protect - are being failed every single day.