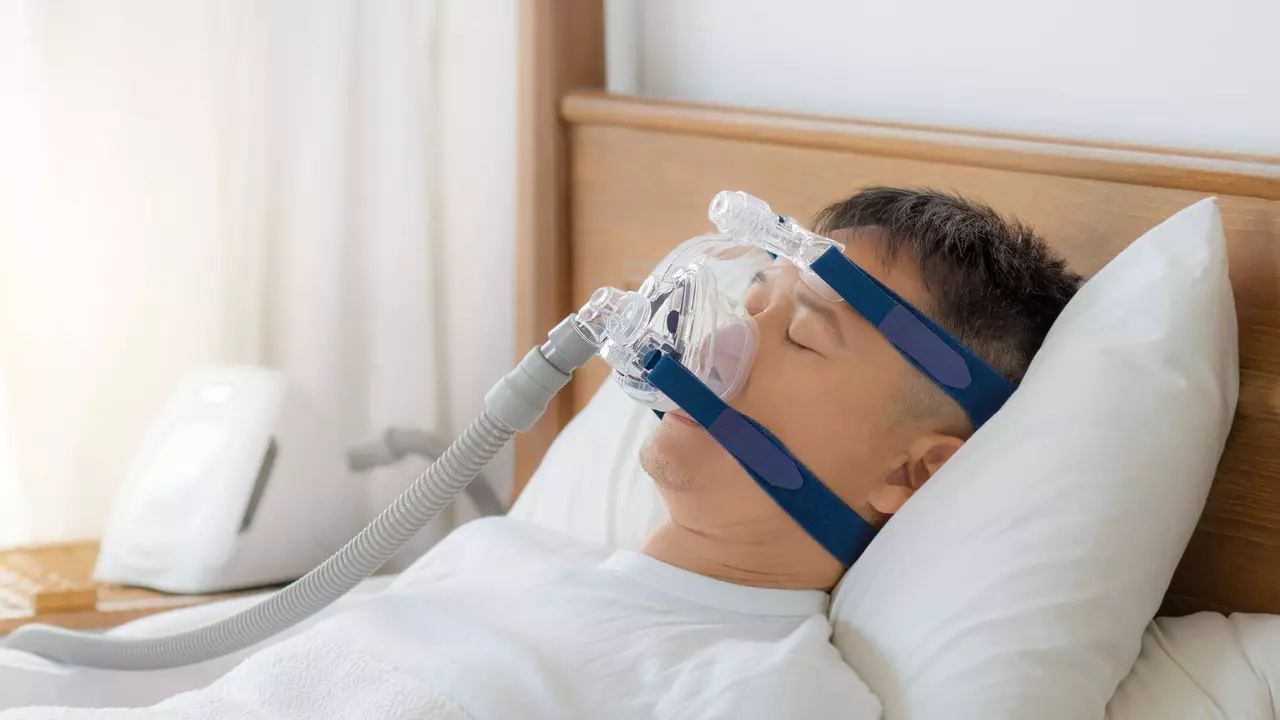
Understanding Sleep Apnea
Sleep apnea is a sleep disorder that is characterized by pauses in breathing or periods of shallow breathing during sleep. These pauses can last from a few seconds to minutes and may occur 30 times or more an hour. This condition is serious and can lead to a number of health problems, including high blood pressure, heart disease, type 2 diabetes, and even stroke. It's also linked to poor performance in everyday activities, such as at work and school, car accidents, and a decreased quality of life.
The Role of Ipratropium Bromide
Ipratropium Bromide is a medication often used to treat symptoms of lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma. It works by relaxing and opening the air passages to the lungs, making it easier to breathe. Given its effectiveness in treating breathing conditions, researchers have started to investigate its potential use in treating sleep apnea.
How Ipratropium Bromide Can Help
There is emerging evidence that suggests that Ipratropium Bromide may be effective in treating sleep apnea. It does so by improving breathing patterns and reducing the number of apneas (pauses in breathing) and hypopneas (shallow breaths) during sleep. This leads to a better quality of sleep and reduced daytime sleepiness, which is often a major problem for people with sleep apnea.
Scientific Studies Supporting Ipratropium Bromide Use
Many scientific studies have been carried out to assess the effectiveness of Ipratropium Bromide in treating sleep apnea. Most of these studies have shown promising results, with significant improvements in breathing patterns and sleep quality. Furthermore, many patients using this medication have reported experiencing fewer side effects, suggesting that Ipratropium Bromide may be a safer treatment option.
Combining Ipratropium Bromide with Other Treatments
While Ipratropium Bromide can be effective on its own, it may be even more effective when used in conjunction with other treatment methods. This could include lifestyle changes, such as weight loss and quitting smoking, as well as the use of other medical devices or medications. Always consult with your healthcare provider to determine the best treatment plan for you.
Understanding the Side Effects
Like all medications, Ipratropium Bromide may have potential side effects. These could include dry mouth, cough, and sinusitis. However, most people who use Ipratropium Bromide do not experience serious side effects. It is important to discuss any potential side effects with your healthcare provider before starting treatment.
Consulting with Your Healthcare Provider
Before starting any new medication, it is important to consult with your healthcare provider. They can provide you with a thorough understanding of the potential benefits and risks, and can help you decide if Ipratropium Bromide is the right treatment for your sleep apnea. Always ensure to discuss any other medications or health conditions you may have, as these could interact with Ipratropium Bromide.
Living with Sleep Apnea
Living with sleep apnea can be challenging, but with the right treatment, it can be managed effectively. Ipratropium Bromide, along with lifestyle changes and other treatments, could help you to achieve a better quality of sleep and improve your overall health and well-being.
Conclusion
In conclusion, Ipratropium Bromide represents a promising potential treatment option for sleep apnea. While further research is needed, current evidence suggests that it can improve breathing patterns and sleep quality, leading to a better quality of life for those suffering from this condition. As always, it is vital to consult with your healthcare provider before starting any new medication or treatment plan.



Joshua Brown
July 6, 2023 AT 17:03Ipratropium bromide, an anticholinergic bronchodilator, has been a cornerstone in COPD management for decades. Its mechanism-blocking muscarinic receptors in the airway smooth muscle-leads to bronchodilation and reduced airway resistance. Recent pilot trials have begun to explore its off‑label use for obstructive sleep apnea. In one crossover study, patients with mild‑to‑moderate obstructive sleep apnea showed a 22 % reduction in apnea‑hypopnea index after nightly nebulized ipratropium. The authors attributed this effect to improved upper airway patency during REM sleep, when cholinergic tone is highest. Moreover, the medication’s short half‑life minimizes systemic exposure, reducing concerns about cardiovascular side effects. Side‑effect profiles in the sleep studies were comparable to placebo, with only mild dry‑mouth reported in less than 5 % of participants. From a pharmacodynamic perspective, the drug’s anticholinergic action may also blunt reflexive airway collapse triggered by carbon dioxide buildup. This hypothesis aligns with animal models where muscarinic blockade dampened pharyngeal muscle tone loss. However, the sample sizes in these early investigations are modest, often fewer than thirty subjects per arm. Consequently, statistical power remains limited, and replication in larger, multi‑center cohorts is essential. Another consideration is the delivery method; nebulized administration during bedtime can be cumbersome for some patients. Alternative formulations, such as metered‑dose inhalers with spacer devices, are being piloted to improve adherence. Clinicians should also evaluate comorbidities-especially glaucoma or urinary retention-before prescribing ipratropium for sleep apnea. Overall, the emerging data suggest a promising adjunctive role, especially for patients who cannot tolerate continuous positive airway pressure. As always, a shared decision‑making conversation with the patient, weighing benefits against practical challenges, is the prudent course of action!
andrew bigdick
July 6, 2023 AT 19:16Hey folks, I’ve been reading up on the ipratropium angle and it’s kinda cool how an asthma drug might double‑duty for sleep apnea. The idea is that it relaxes the airway muscles just enough to keep the airway open when you’re snoring. I’d still say CPAP is the gold standard, but having a backup drug could be handy for mild cases. Also, it’s cheap and widely available, which is a plus for people without insurance. Just make sure you chat with your doc before adding it to your bedtime routine.
Shelby Wright
July 6, 2023 AT 21:29Honestly, this sounds like a pharmaceutical PR stunt to push another pill on desperate sleepers.
Ellen Laird
July 6, 2023 AT 23:43While your cynicism is noted, the pre‑existing literature definatly warrants a more measured appraisal.
rafaat pronoy
July 7, 2023 AT 01:56Interesting read! I’m not a medical pro, but I’ve tried ipratropium for my asthma and it helped with nighttime breathing. Maybe it could work for apnea too? :) Just curious, does anyone know if there’s a specific dosage for sleep‑related use?
sachin shinde
July 7, 2023 AT 04:09First, you should not conflate “asthma” treatment dosage with “sleep apnea” protocols; the two indications have distinct pharmacokinetic targets. Second, the literature you referenced lacks a randomized controlled trial, which is the gold standard for efficacy assessment. Third, using emoticons in a scientific discourse undermines credibility. Finally, consult a pulmonologist before self‑medicating.
Leon Wood
July 7, 2023 AT 06:23Great points raised about the need for more research-let’s keep the conversation moving! If ipratropium does prove helpful, it could give patients another tool besides CPAP, which some find uncomfortable. Think of the potential to improve daily energy levels and overall quality of life. It’s exciting when a drug finds a new purpose beyond its original label. Keep sharing updates, and we’ll all benefit from a broader evidence base. Stay hopeful, everyone!
George Embaid
July 7, 2023 AT 08:36Absolutely, the cross‑disciplinary collaboration between sleep specialists and pulmonologists can accelerate these findings. It also highlights the importance of cultural sensitivity when discussing treatment options across diverse populations. Let’s encourage open‑minded dialogue and shared learning.
Meg Mackenzie
July 7, 2023 AT 10:49What they don’t tell you is that big pharma already has a stake in the CPAP market, so they’re quick to downplay cheap alternatives. Ipratropium is off‑patent, which means they lose money if it becomes mainstream for sleep apnea. Some “studies” are funded by device manufacturers, skewing the results toward positive CPAP outcomes. That’s why it feels like the data is always a little too polished. Keep an eye on who sponsors the trials. The truth is often hidden behind corporate press releases.
Shivaraj Karigoudar
July 7, 2023 AT 13:03Indeed, the entanglement of financial incentives with clinical research outcomes is a well‑documented phenomenon in the healthcare economics literature. When we examine the funding pipelines, we observe that device manufacturers frequently allocate grants to institutions that have an established CPAP program, thereby creating a feedback loop of positive reinforcement. This systemic bias can manifest as publication bias, where negative or inconclusive results regarding pharmacologic alternatives are less likely to reach peer‑reviewed journals. Moreover, the regulatory landscape for repurposed drugs like ipratropium is comparatively lax, which may accelerate off‑label adoption without rigorous phase III validation. From a pharmacovigilance standpoint, the lack of large‑scale real‑world data hampers our ability to conduct post‑marketing surveillance for rare adverse events. It is also worth noting that patient adherence to nebulized therapy often suffers due to the logistical burden of nightly device setup, a factor that is sometimes under‑reported in efficacy trials. In clinical practice, the integration of a bronchodilator into a sleep regimen must consider comorbidities such as glaucoma, where anticholinergic side effects could be detrimental. Additionally, the heterogeneity of apnea phenotypes-obstructive, central, mixed-means that a one‑size‑fits‑all pharmacologic approach is defintely impractical. Nonetheless, the mechanistic rationale-attenuation of cholinergic-mediated airway collapse-is biologically plausible and merits further investigation. Future randomized controlled trials should stratify participants by apnea severity and phenotype, employ blinded outcome assessment, and report both polysomnographic metrics and patient‑reported outcomes. Only through such comprehensive methodology can we disentangle genuine therapeutic benefit from commercial hype. Until then, clinicians must exercise judicious clinical judgement, balancing the allure of inexpensive interventions against the robustness of the evidence base. Finally, transparency in conflict‑of‑interest disclosures remains paramount to maintaining scientific integrity.
Matt Miller
July 7, 2023 AT 15:16Does anyone have data on the optimal timing of ipratropium dosing relative to sleep onset?
Fabio Max
July 7, 2023 AT 17:29Good question! Some protocols suggest administering the dose 30‑45 minutes before bedtime to align peak plasma concentration with the early night period, but the evidence is still emerging.
Darrell Wardsteele
July 7, 2023 AT 19:43First off, the spelling in many of these posts is atrocious; proper English is a matter of national pride. Second, the claim that ipratropium is “cheap” ignores the fact that imported formulations can be priced exorbitantly in our country. Third, any discussion that sidesteps the role of American manufacturers in supporting domestic research is disingenuous. Fourth, let’s keep the conversation factual and free from baseless conspiracy fluff. Finally, I urge everyone to cite reputable sources when making medical assertions.
Madeline Leech
July 7, 2023 AT 21:56Look, you sound like you’re defending big‑pharma without any evidence-just a typical nationalist rant. The safety profile of ipratropium is well documented, and dismissing peer‑reviewed studies as “fluff” shows a lack of critical thinking. If you want to argue, bring real data, not patriotic slogans.
Barry White Jr
July 8, 2023 AT 00:09IPR helps some with apnea.
Andrea Rivarola
July 8, 2023 AT 02:23The evolution of therapeutic strategies for sleep‑related breathing disorders reflects a broader trend toward personalized medicine, yet many patients remain tethered to a one‑size‑fits‑all approach. Historically, continuous positive airway pressure devices have dominated the treatment landscape, offering mechanical splinting of the upper airway during sleep. While effective for many, adherence rates hover around 50 %, underscoring the need for alternative or adjunctive therapies. In this context, the repurposing of ipratropium bromide presents an intriguing pharmacologic avenue, especially given its well‑established safety profile in chronic obstructive pulmonary disease. The anticholinergic action of ipratropium can theoretically reduce airway collapsibility by attenuating vagal tone, a hypothesis supported by a handful of pilot studies. However, these investigations are limited by small sample sizes and heterogeneous patient populations, which hampers the generalizability of their findings. Moreover, the delivery mechanisms-ranging from nebulizers to metered‑dose inhalers-introduce variability in drug deposition within the upper airway, a factor that has not been thoroughly quantified. Ethical considerations also arise when clinicians prescribe off‑label medications without robust evidence, as patient autonomy and informed consent become paramount. From a health economics perspective, a low‑cost oral or inhaled agent could alleviate the financial burden associated with CPAP equipment, especially in resource‑constrained settings. Yet, cost‑effectiveness analyses are scarce, and the potential for increased healthcare utilization due to side‑effects, albeit rare, must be accounted for. Patient preferences further complicate the equation; some individuals might favor a nightly inhalation routine over a device, while others may find any additional step burdensome. The heterogeneity of apnea phenotypes-obstructive versus central-also demands tailored interventions, suggesting that ipratropium may be more suitable for specific subgroups. Future research should therefore prioritize stratified trial designs, robust blinding, and patient‑reported outcome measures to capture real‑world efficacy. Until such data emerge, clinicians must navigate a delicate balance between innovation and evidence‑based practice. Ultimately, fostering interdisciplinary collaboration among sleep physicians, pulmonologists, and pharmacologists will be key to unlocking the full therapeutic potential of agents like ipratropium.
Tristan Francis
July 8, 2023 AT 04:36All this talk about trials sounds like a cover‑up; the big guys don’t want cheap drugs to replace their pricey machines.
Keelan Walker
July 8, 2023 AT 06:49Wow this thread is buzzing with ideas 😊 ipratropium could be a game changer for some 😮 the fact that it’s already on the market makes it super accessible 🙌 we need more real world data but the early signs are promising 🌟 if you combine it with lifestyle tweaks like weight loss you might see bigger benefits 🏃♀️ keep the discussion alive and share any personal experiences 🗣️
Heather Wilkinson
July 8, 2023 AT 09:03Love the optimism! 🌈 It’s true that a multi‑pronged approach often yields the best outcomes. Thanks for bringing the positivity! 👍
Henry Kim
July 8, 2023 AT 11:16I’ve been quietly reading the studies and think the evidence, while promising, still needs larger trials before wide adoption.